Regulatory pressure continues to increase on medical device manufacturers. UDI requirements have taken hold, you need a partner that can keep you up to date on the regulations and provide you with complete solutions that will meet federal standards. Medical Device labeling is included under FDA Title 21 – Chapter I – Subchapter H.
Dasco brings complete medical device solutions to you, including, labeling software, printers (single and full color), labels (blank and printed), rounding it out with full support to ensure you get the system installed and running to meet standards. Dasco specializes in on-demand printing giving you full control over your label designs, reducing lead times, inventory and obsolescence.
There are many things to consider when choosing a label printer to meet your needs. Label size, label material, printing volume, print quality, color options, and cost of ownership are some major factors to consider. The main types of label printers include direct thermal, thermal transfer, ink jet or inline laser. It is best to compare the options to provide you with the capabilities you need yielding the lowest cost of ownership. Consult with a Dasco printing professional to review the options that will meet your needs.

The FDA requires all medical equipment be clearly labeled with the proper information and accessible by anyone using the equipment so electing the proper label material is the most critical part of your identification journey. Depending upon your application there are many materials you can select, is this a device label, equipment label, packaging label for a bag or box. Does the label need to meet UL, CSA, CE standards or will it be exposed to cleaning solutions or temperature extremes? The wrong material can lead to the printing being removed or the label coming off resulting in your device being considered “misbranded” by the FDA. There are hundreds of material and adhesive combinations to meet the wide variety of applications in the Medical Device market. Work with your Dasco label specialist to evaluate label material options that will meet your specific needs.
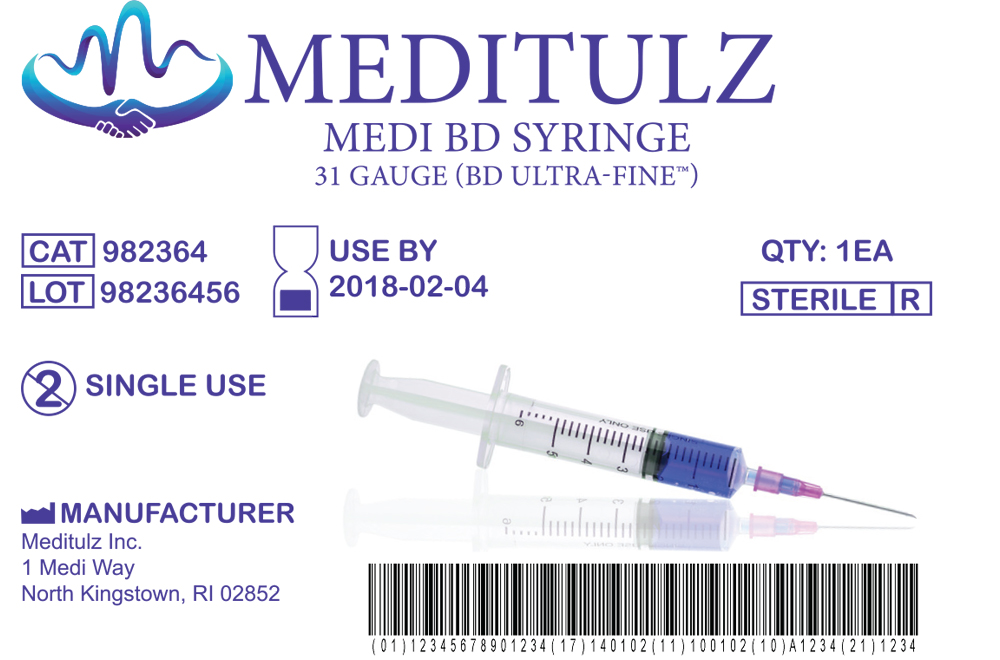
Full control over your labeling process begins with a modern Label Management System (LMS). The right LMS allows you to manage the complexity of increasing regulatory compliance. Implementing an LMS not only allows you to create labels with the necessary UDI device identifiers, they allow you to store labels, integrate with external data sources, document/track changes and maintain print history to meet regulatory requirements. The right LMS will allow you to improve quality, comply with increasing regulations and reduce costs.





